Give reasons:
n-Butyl bromide has higher boiling point than t-butyl bromide.
The boiling point of n-butyl bromide is higher than that of t-butyl bromide because n-butyl bromide is a straight chain molecule having larger surface area and therefore, has stronger intermolecular forces. On the other hand, t-butyl bromide is branched molecule, so it has a smaller surface area. Hence, it has weaker intermolecular force. Thus, n- Butyl bromide has higher boiling point than t-butyl bromide.
Iodoform test is not given by :
HCHO
CH3CHO
CH3COCH3
C2H5OH
A.
HCHO
Iodoform test is given by those compounds which have -CO-CH3 gorup or on oxidation yields this group.
Formaldehyde does not have -CO-CH3 group. It does not give iodoform test.
Give reasons:
Racemic mixture is optically inactive.
The racemic mixture contains two enantiomers (d and l forms) in equal proportions and thus, the rotation due to one isomer is cancelled by the rotation due to another. Therefore, it has zero optical rotation and hence, it is optically inactive.
Pure methane can be produced by
Wurtz reaction
Kolbe's electrolytic method
soda lime decarboxylation
reduction with H2
C.
soda lime decarboxylation
Methane cannot be produced by Wurtz reaction, Kolbe's electrolytic method and reduction with H2 because, it has one carbon atom. Pure methane can be produced by the decarboxylation of sodium acetate.
Which would undergo SN2 reaction faster in the following pair and why?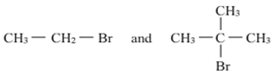
Sn2 reactions are bimolecular with simultaneous bond-making and bond-breaking steps. Primary alkyl halides prefer to undergo SN2 reactions than tertiary alkyl halides because of less steric hindrance experienced by the approaching nucleophile.
Hence, out of the given pair, (CH3 –CH 2 – Br ) would undergo SN2 reaction faster.
