Answer the following:
(i) Haloalkanes easily dissolve in organic solvents, why?
(ii) What is known as a racemic mixture? Give an example.
(iii) Of the two Bromo derivatives, C6H5CH(CH3)Br and C6H5CH(C6H5)Br, which one is more reactive in Sn1 substitution reaction and why?
i) Haloalkanes can easily dissolve in organic solvents of low polarity because the new forces of attraction set up between haloalkanes and the solvent molecules are of same strength as the forces of attraction being broken.
(ii) A mixture of equal amounts of two enantiomers is known as racemic mixture. For example: When a 3° halide undergoes substitution with KOH, the reaction proceeds through SN 1 mechanism forming the racemic mixture in which one of the products has the same configuration as a reactant, while the other product has an inverted configuration.
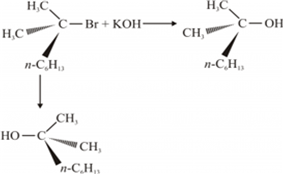
(iii) In SN1 reaction mechanism leads through the carbocation pathway.
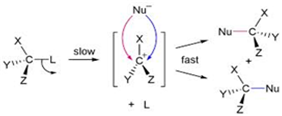
More stable the carbocation, more reactive will be substrate .the carbocation formed by two compounds are as follows:
Compound (C6H5)2CHBr = carbocation (C6H5)2CH+
Compound C6H5CH(CH3)Br=carbocation C6H5(CH3)CH+
Out of these two carbocations (C6H5)2CH+ is more stable than C6H5(CH3)CH+ because the carbocation(C6H5)2CH+ is resonance stabilised by two benzene rings .therefore (C6H5)2CHBr is more reactive than C6H5CH(CH3)Br. Resonance stabilisation of carbocation with one benzene ring is shown :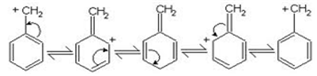
The reaction of propene with HOCl(Cl2+H2O) proceeds through the intermediate:
CH3−CH+−CH2−Cl
CH3−CH(OH)−C+H2
CH3−CHCl−C+H2
CH3−CHCl−C+H2
A.
CH3−CH+−CH2−Cl
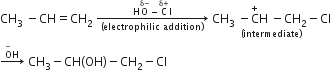
In SN2 reactions, the correct order of reactivity for the following compounds CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is
CH3Cl > (CH3)2CHCl >CH3CH2Cl > (CH3)3CCl
CH3Cl > CH3CH2Cl > (CH3)2CHCl >(CH3)3CCl
CH3CH2Cl > CH3Cl >(CH3)2CHCl >(CH3)3CCl
CH3CH2Cl > CH3Cl >(CH3)2CHCl >(CH3)3CCl
B.
CH3Cl > CH3CH2Cl > (CH3)2CHCl >(CH3)3CCl

The synthesis of alkyl fluorides is best accomplished by
free radical fluorination
Sandmeyer's reaction
Finkelstein reaction
Finkelstein reaction
D.
Finkelstein reaction
Alkyl fluorides can be prepared by the action of mercurous fluorides or antimony trifluoride (inoragnic fluorides) on corresponding alkyl halide.
This reaction is known as Swarts reaction
CH3Br + AgF → CH3F + AgBr
But, when the action of NaI/acetone takes place on alkyl chloride or bromide alkyl iodide forms. This reaction is called 'Finkelstein reaction.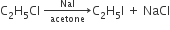
Free radical fluoridation is a highly explosive reaction. So not preferred for the preparation of fluoride.
