A photo-chemical rection may be defined as a process or reaction which is initiated by light absorption.
A reaction between H2 gas and chlorine gas is a photo-chemical reaction. The proposed mechanism for this reaction is given as follows:
Primary Process:
Secondary Process:
(step II)
(step III)
Step II and III are called chain propagation steps. The chain termination takes place on the walls of the reaction vessel by the reaction.
(i) Define specific reaction rate.
(ii) Define Half-life period of a chemical reaction. Also obtain the expression for half-life period.
Or
Derive the general for of the expression for the half-life of a first order reaction.
(i) Specific reaction rate. This is also called rate constant or velocity constant. Specific reaction rate may be defined as the rate of reaction under specific conditions, when the product of concentration of the reactants is unity.
(ii) Half-life period of a chemical reaction in which the concentration of reactant is reduced to half of the intial value of concentration.
It is denoted by tl/2 or t0.5.
The rate equation for a reaction of first order is expressed as:
or
When
On substituting these values, we get
The relation (i) is the required expression for half-life period of first order reaction.
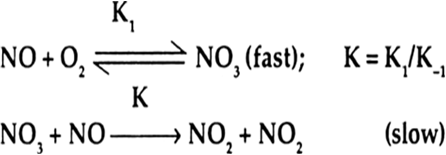
The slow step is the rate determining step. In the slow step in this reaction, 1 molecule of NO3 (intermediate) and 1 molecule of NO combine to form the products. Therefore, rate of this reaction depends upon 1 concentration term of NO3 and 1 concentration term of NO.
∴ Molecularity of the reaction = 1 + 1 = 2.
Rate = K [NO3] [NO]
NO3 is an intermediate which is formed rapidly by the collision of 1 molecule of NO and 1 molecule of O2.
[NO3] ∝ [NO][O2]
Rate = K1 [NO3] [NO]
= K [NO] [O2] [NO]
= K [NO]2[O2]
Order of reaction is 2 + 1 = 3.
