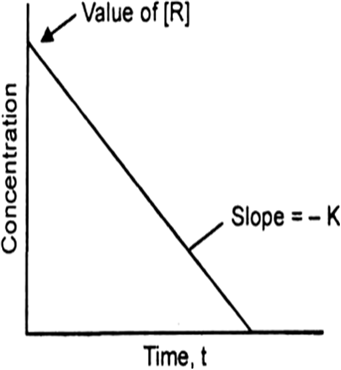
Answer the following questions on the above curve for a first order reaction A → P.
(a) What is the relation between slope of this line and rate constant.
(b) (i) Calculate the rate constant of the above reaction if the slope is 2 x 10–4 s–1.
(ii) Derive the relationship between half life of a first order and its rate constant.
(a) Slope = – k
or k = – 2 x 10–4 mol–1 Ls–1.
(ii) Consider the following first order reaction
A → P
at t = 0 a 0
at t = t (a – x) x
Suppose a is the initial concentration of reactant. After time t, x gm mol lit–1 is changed to P. According to law of mass action the rate of reaction at time t is directly proportional to the concentration of A at that instant i.e., (a – x)
Hence
is the rate and K is the rate constant, after rearranging the above equation, we get
...(i)
On integrating the above equation
We get
When t = 0, x = 0 hence – In a = constant substituting this value of constant in eqn. (ii) we get
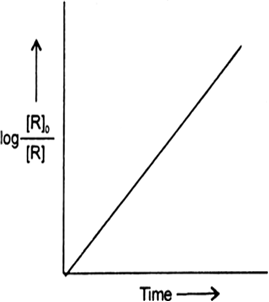
For a certain chemical reaction variation in the concentration in [R] vs time(s) plot is given below:
For the reaction write/draw
(i) What is the order of the reaction?
(ii) What are the units of rate constant K?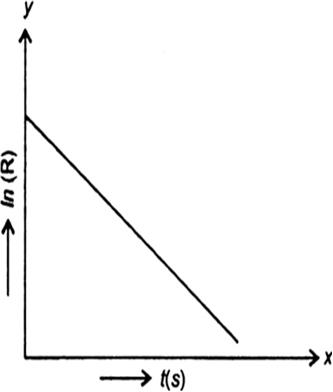
(iii) Give the relationship between K and t1/2 (half-life period).
(iv) What does the slope of the above line indicate?
(v) Draw the plot Vs. time(s).
Or
For a certain chemical reaction:
The experimentally obtained information is tabulated below:
The experimentally obtained information is tabulated below :
|
Exp. |
[A]0 |
[B]0 |
Initial rate of reaction |
|
1 2 3 4 |
0.30 0.60 0.30 0.60 |
0.30 0.30 0.60 0.60 |
0.096 0.384 0.192 0.768 |
For this reaction:
(i) Derive the order of reaction w.r.t. both the reactants A and B.
(ii) Write the rate law.
(iii) Calculate the value of rate constant K.
(iv) Write the expression for the rate of reaction in terms of A and C. 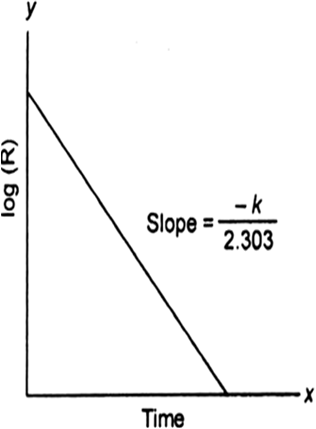
For the reaction: A → B.
Let a is the initial concentration of A in g moles L–1 and (a – x) is the concentration in g moles L–1 after time t, then according to law of mass action
Rate of reaction ![]() (a - x)
(a - x)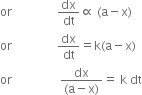
Integrating the above equation, we get
![]()
when ![]()
or ![]()
or ![]()
or ![]()
Changing natural log to base 10, we get
![]()
Nature of the curve: Hypothetical variation of conc. of reactant [R] and product [P] during the course of reaction.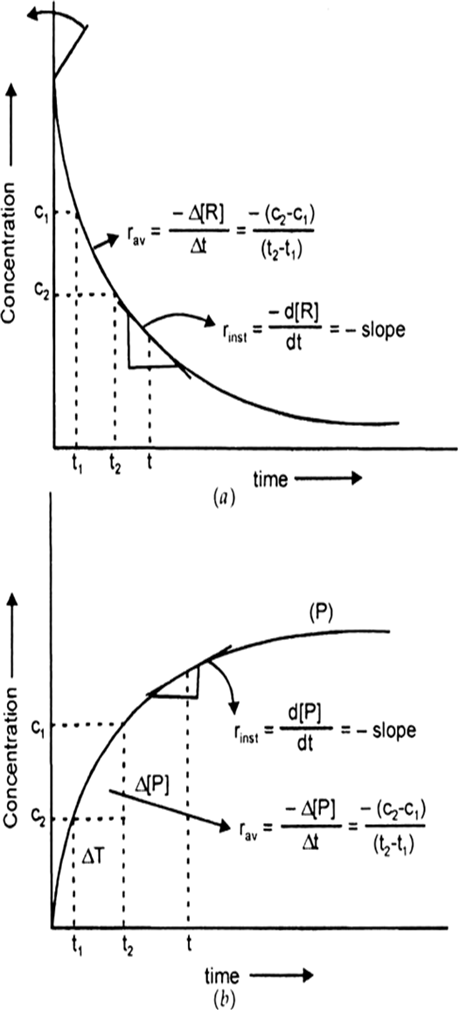
Fig. Instantaneous and average rate of a reaction.
