The change in free energy is equal to the useful work done in a reversible process at constant temperature and pressure
ΔG = Wnet ...(i)
In an electrochemical cell and work obtained is equal to the charge transferred multiplied by the potential difference:
Welectrical = – nFE ...(ii)
The negative sign appears because the work is done by the charge. In equation (ii) n is the moles of electrons gained or lost in redox reaction and F is the Faraday constant.
In a cell when only electric work is done, then
Wnet= WeIectrical = – nFE ...(iii)
From relation (i) and (iii), we have
ΔG = – nFE
or ΔG = – mFE°cell.
Zn/Zn2+(0.1 M) || Cd2+ (0.01) | Cd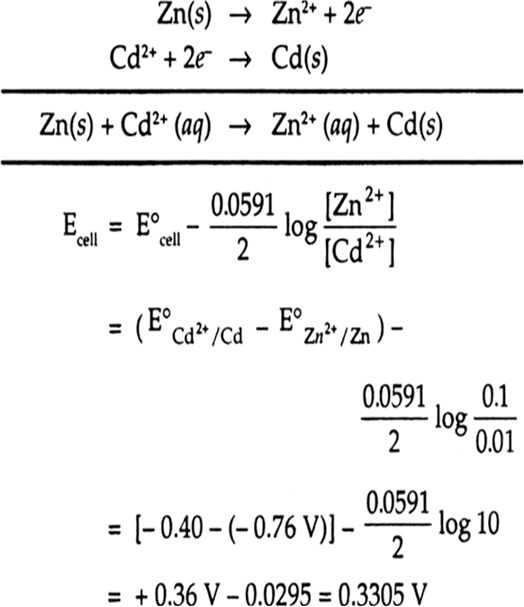
The following curve is obtained when molar conductivity λm (y-axis) is plotted against the square root of concentration C1/2 (x-axis) for two electrolytes A and B.
(a) What can you about the nature of the two electrolytes A and B.
(b) How do you account for the increase in molar conductivity λm for the electrolytes A and B on dilution.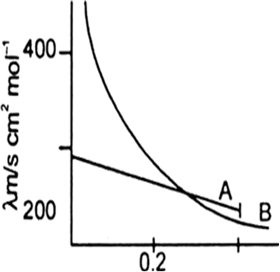
(a) A is strong electrolyte and B is weak electrolyte.
(b) The molar conductivity of a strong electrolyte:
(A) increase slightly as the concentration increased. This is because greater inter ionic attractions retrad the motion of the ions therefore molar conductivity decreases.The molar conductivity of a weak electrolyte.
(B) increase with decrease in the concentration of the solute in solution. The increase is very rapid at lower concentrations. The increase in the molar conductance with dilution is due to an increase in the effective degree of ionisation (α) at lower concentration and release of more full ions in the solution.

(i) The relation between free energy change and emf of a galvanic cell of the reaction is given as
ΔG = – nFEcell
(ii) The relation between free energy change and equilibrium constant of the reaction is given by
ΔG = – 2.303 RT log Kc.
