Cu2+ + 2e– → Cu E° = + 0.34 V
Ag+ + 1e– → Ag E° = + 0.80 V
(i) Construct a galvanic cell using the above data.
(ii) For what concentration of Ag+ ions will the emf of the cell be zero at 25°C, if the concentration of Cu2+ is 0.01 M ? [log 3.919 = 0.593]
(i) Cu(s) | Cu2+ (aq) || Ag+ (aq) | Ag(s)
(ii) Cu(s) | Cu2+ (aq) || Ag+ (aq) | Ag(s)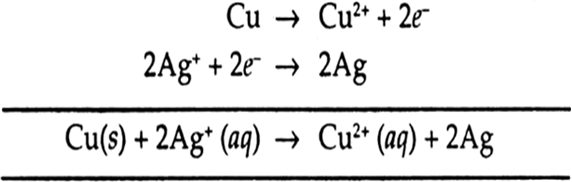


m = Z x I x t
or
or
or
or
(i) Resistance (k) =
Conductance (C) =
=
Specific conductance (k)
= Conductance x cell constant
(ii) Molar conductance (C)
Molar conductance
