Λ°m (CH3COOH) = λ°H+ + λ°CH3coo–
= Λ°m(HCl) = λ°m (CH3COONa) – λ°NaCl
= 425.9 + 91.0 –126.4
= 390.5 s cm2 mol–1.
We have given that
m = 127 gm,
I = 50 A
Z = 0.0003294 g/c
t = ?
By Faraday's first law,
m = ZIt
or
t = 77118 sec.
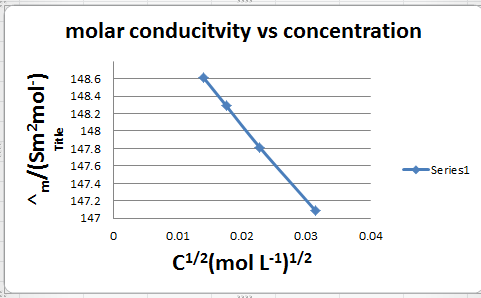
|
C/mol L–1 |
Λ/s cm2 mol–1 |
|
0.000198 0.000309 0.000521 0.000989 |
148.61 148.29 147.81 147.09 |
|
C1/2 /(mol L–1)1/2 |
A/s cm2 mol–1 |
|
0.01407 0.01758 0.02283 0.03145 |
148.61 148.29 147.81 147.09 |
We have given that
current = 0.5 ampere
weight of H2 (W) = ?
t = 1 hour = 3600 sec,
Z = 0.00001.
W = Z x c x t
= 0.00001 x 0.5 x 3600 g = 0.018 g
2g or H2 at STP occupies = 22.4 litres
0.018 g of H2 at STP occupies
Hence, Volume of H2 produced at STP = 0.2016 litres.
