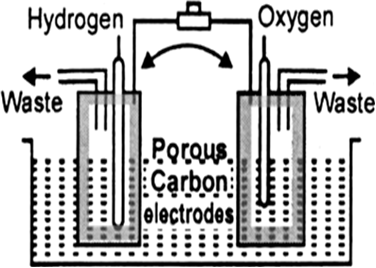
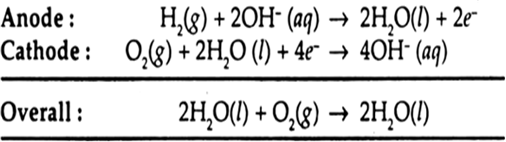
Advantages: (i) Fuel cells are efficient and free from pollution.
(ii) The only product in the reaction of fuel cell is water which can be removed and the astronauts of a spacecraft can drink it.
Equivalent conductivity : The conductivity of a volume (V) of a solution containing one equivalent of electrolyte placed between two electrodes separated by unit distance apart and of large enough area of cross-section to hold the entire volume (V) is called equivalent conductance. It is denoted by Λeq.
Λeq = k x V
where k = Specific conductance
V = Volume of solution containing one equivalent of electrolyte.
Molar conductivity : It is the conductivity of volume (V) of a solution containing 1 mole of a dissolved electrolyte place between two electrodes separated by unit distance apart and of enough area of cross-section to hold the entire volume V. It is denoted by Λm.
Λm= k x V = k / V where V = Volume of solution Containing 1 mole of electrolyte C = molarity of solution k = Specific conductivity.
What is corrosion? Describe the electrochemical phenomenon of rusting of iron.
Corrosion is the process of slowly eating away of the metal due to attack of the atmospheric gases on the surface of the metal resulting into the formation of compounds such as oxides, sulphides, carbonates, etc.
The corrosion of iron is called rusting.
According to theory of rusting, impure iron surface behaves as a small electrochemical cell in the presence of water containing dissolved oxygen or CO2.
The pure iron acts as anode and impure surface as cathode.
At Anode : Iron atom undergo oxidation spontaneously forming Fe2+ ion.
Fe → Fe2+ (aq) + 2e- E°cell = – 0.44 V
Fe2+ ions move into solution and electrons into cathodic area where they are picked up by H+ ions of the solution.
At cathode:
H+ ions are produced by secondary reaction either from H2O or from H2CO3 (CO2 + H2O)
H2O ---->H+ + OH–
H2CO3------> H+ + HCO3–
The overall reaction of the corrosion cell may be represented as:
The Fe2+ ions move through water and come at the surface where these are further oxidized into Fe3+ ions by atmospheric oxygen to form hydrate ferric oxide known as rust, Fe2O3.xH2O.
