Answer:
(i) Mn2+ is more stable than Mn3+ because Mn+2 has exactly half-filled orbitals, while Fe+2 after losing one e– half-filled orbitals.
(ii) Ce+4 achieve inert gas structure of Xenon, to require extra stability.
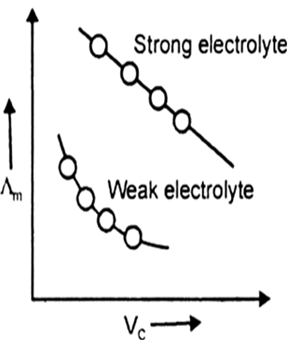
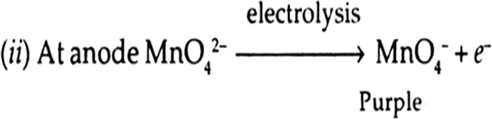
Answer:
The advantage of water fuel cell over ordinary cell is,
(i) It has high-efficiency and eco-friendly.
(ii) The H2O produced can be used by astronauts for drinking purposes.
