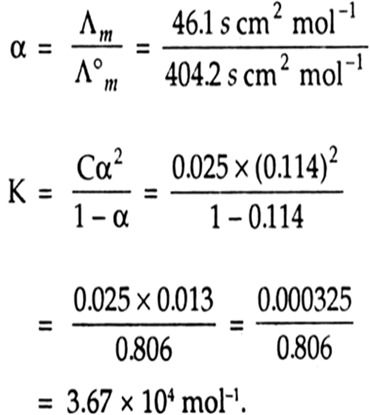
The conductivity of a solution is directly proportional to number of ions present in a unit volume of the solution because current is carried forward by the ions. With dilution number of ions in unit volume decreases so that conductivity also decreases. Hence with dilution conductivity decreases.

 Switch
Switch