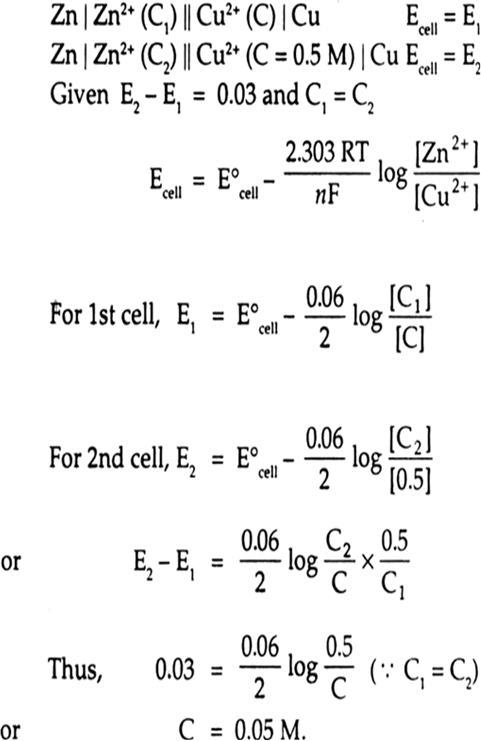
The potential of a hydrogen electrode in a solution of unknown [H+] is 0.29 V at 298 k measured against a standard hydrogen electrode. Calculate the pH of the solution.
The both electrode are same thus there =0
Since the potential of the hydrogen electrode measured against standard hydrogen electrode is a positive value, the electrode is acting as the anode. If the unknown [H+] be x, the Nernst equation takes the form
Since both electrodes are the same,
∴
or
Since
or
124 Views