Answer:
According to Raoult's law the partial vapour pressure of each volatile compound in any solution is directly proportional to its mole fraction
if the vapour pressure is higher then solution is said to exhibit positive deviation
Positive deviation is shown by ethanol and water, cyclohexane and ethanol, acetone and diethylether etc.
Reasons:
(i) A—B interaction is weaker than A—A or B—B in positive deviation.
If the vapour pressure is lower then solution then solution is said to exhibit negtive deviation.
Negative deviation is shown by chloroform and acetone, methanol and acetic acid, H2O and HCl, H2O and HNO3 etc.
Reason:
(ii) A—B interaction is stronger than A—A or B—B in negative deviation.
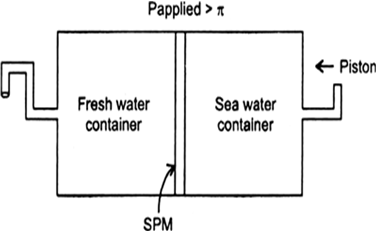
(i) Name the process occurring in the above plant.
(ii) To which container does the net flow of solvent take place?
(iii) Name one SPM which can be used in this plant.
(iv) Give one potential use of the plant.
Answer:
(i) Reverse osmosis.
(ii) Pure water is forced out of the solution to pass through the pores of the membrane in the opposite direction.
(iii) Parchment or cellophone.
(iv) This process is used in desalination to get salt-free water from sea water.
Solubility of a substance is its maximum amount that can be dissolved in a specified amount of solvent at a specified temperature. It depends
upon the nature of solute and solvent as well as temperature and pressure. In general, a solute dissolves in a solvent if the intermolecular interactions are similar in the two or we may say like dissolves like.
