
Answer: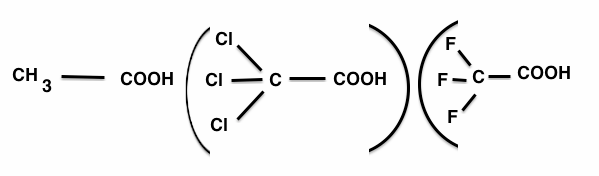
Among H,Cl and F, Hydrogen is least electronegtive while F is most electronegtive than Cl and H.
Thus F can withdraws more electron towards itslef more than Cl and H. So trifluoroacetic acid can easily lose the H+ ions. i.e.
trifluroacetic acid ionize to the larger extent .
Now more the ion produces the greater is the dpress ion of the freezing point
Hence, the depression of freezing point increase in order :
Acetic acid<trichloracetic acid < trifluroacetic acid
Answer:
Mol. mass of benzoic acid, C6H5COOH
= 6 x 12 + 5 x 1 + 12 + 16 + 16 + 1
= 72 + 5 + 12 + 16+16 + 1
= 122 g mol–1
by using formula;
here x= amount of substance required
Amount of benzoic acid required
Answer:
number of moles present in 92g of Na+ ion.
=92/23g mol-1 = 4mol
Molality,
No. of gm moles of solute = 92/23 = 4
Wt. of water (solvent) = 1 kg
