Mole of benzene
Mass of Toluene
Mole fraction of benzene
Answer:
Let us calculate the concentration of the first solution with osmatic pressure 4.98bar
mass of glucose = 36g
molar mass of gulcose = 180g/ mol
therefore number of moles of gulcose = 36/180
= 0.2 moles
volume of the solution = 1L
molarity = No. of moles of glucose / vol.of solution
molarity =0.2/1L
C1 = 0.2 moles/L
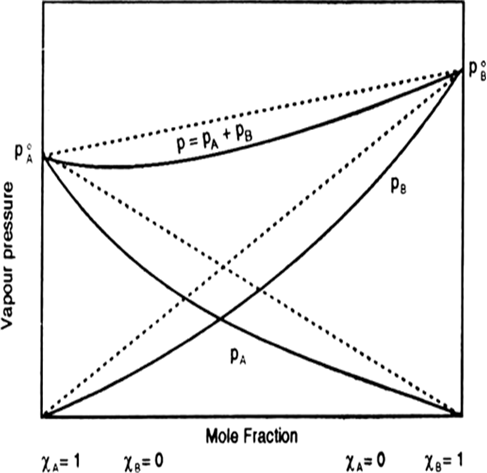
Vapour pressures of pure acetone and chloroform at 328 k are 741.8 nm Hg and 632.8 mm Hg respectively. Assuming that they form ideal solution over the entire range of composition, plot Ptotal , Pchlroform and Pacelone as a function of Xactone. The experimental date observed for different composition of mixture is:
|
100 x xacetone |
0 |
11.8 |
23.8 |
36.0 |
50.8 |
58.2 |
64.5 |
72.1 |
|
Pacetone / mm Hg
|
0 |
54.9 |
110.1 |
202.4 |
327.7 |
405.9 |
454.1 |
521.1 |
|
Pchloroform/ mm Hg |
632.8 |
548.1 |
469.4 |
359.7 |
257.7 |
193.6 |
161.2 |
120.7 |
Plot this data also on the same graph paper, indicate whether it has positive deviation or negative deviation from the ideal solution.