
Solution :
given that,
pressure of CO2=2.5atm
1 atm = 1.01325x 105 pa so that
pressure of CO2 = 2.5x1.01325x105pa
= 2.533125 x 105 pa
KH = 1.67 x 108 pa
ACcording to henry's law p= KH*X
or X=P/KH
= 2.533125 x 105/1.67x 108
= 1.52 x 10-3
But we have 500ML odf soda water so that
Volume of water = 500mL
Density of water =1g/ml
mass = volume x density
500 mL of water = 500g of water
molar mass f water (H2O) = 18g mol-1
number of moles =
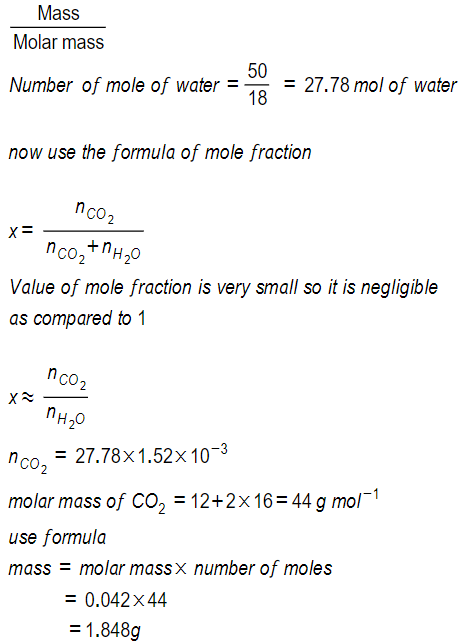
mole fraction of liquid b = 1-0.30 = 0.70
It is given that the solubility of H2S in water at STP is 0.195m, i.e., 0.195 mol of H2S is dissolved in1000 g of water.
=
At STP pressure (P) = 0.987bar
According to henry's law p = kH x
Vapoure pressure of pure water (solvent) at 298 K, p0 = 23.8 mm
Vapour pressure of solution, p = ?
Mass of solvent ,W = 850 g
Mass of solute,M = 50 g
Mol. mass of water (H2O), M = 18 g mol–1
Mol.mass of urea NH2 CO NH2
= 14 + 2 + 12 + 16 + 14 + 2
= 60 g mol–1
According to Raoult's law,
Hence, 23.78 mm Hg. Ans.
