Answer:
Raoult established that the lowering of vapour pressure depends only on the concentration of the solute particles and it is independent of their identity.
establishes a relation between vapour pressure ofthe solution, mole fraction and vapour pressure of the solvent, i.e.
Answer:
Raoult’s law states that the partial vapour pressure of a component of a solution at a given temperature is equal to the product of the vapour pressure of the pure component at that temperature and its mole fraction in the solution.
Positive Deviation from Raoult’s law: In those non-ideal solutions, when partial pressure of component ‘A’ in the mixture of ‘A’ and ‘B’ is more than that calculated from Raoult’s law. Similarly, the partial vapour pressure of component ‘B’ can be higher than calculated from Raoult’s law. This type of deviation from ideal behaviour is called positive deviation from Raoult’s law, e.g., water and ethanol, chloroform and water, ethanol and CCl4, methanol and chloroform, benzene and methanol, acetic acid and toluene, acetone and ethanol, methanol and H2O.
For positive deviation ΔHmixing > 0.
Answer:
Vapor pressure or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system.
(a) When a volatile solute is dissolved in a liquid total vapour pressure is given by the sum of total vapour pressure of both the solvent and solute ie.,
P = PA + PB.
(b) When a non-volatile solute is dissolved in a liquid, the vapour pressure of liquid decreases and this lowering of vapour pressure is proportional to the mole fraction of solute added to it.
If the non volatile solute is present in the solution, the vapour pressure of the solution depends only upon the solvent. If the vapour pressure of the solution is P and the partial vapour pressure of solvent is PA then P = PA
(Because solute has no partial vapour pressure due to non-volatile nature)
or ![]()
where P0A is the vapour pressure of pure solvent and xA and xB are the mole fractions of solvent and solute respectively.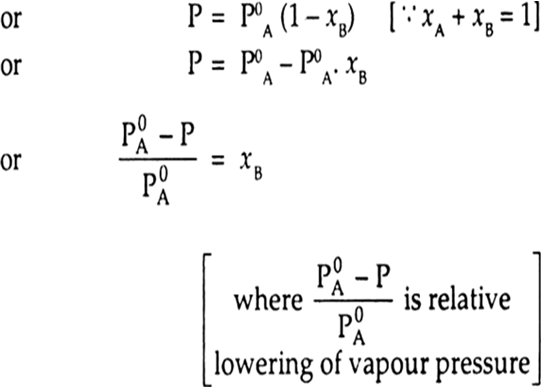
Answer:
The colligative properties of solution depend on the total number of solution.
since the eletrolytes ionise and give more than one particle per formula unit in solution the colligative effect of an eletrolyte solution is alaways greater than that of a non electrolyte of the same molar concentration.
The molar mass of acetic acid in a solution of benzene solvent is greater than its formula molar mass because two CH3COOH molecules form a dimer (CH3COOH)2 in solution. But we know that the molar mass is defined as the mass of 6.023 x 1023 particles of the substance. In case of a normal solute like CH3COOH, the molar mass of acetic acid is equal to the mass of 6.023 x 1023 molecules of formula CH3COOH. While in solution the solution dimerises and becomes a bigger molecule like (CH3COOH)2. Now in this case the molar mass of the solute is equal to the mass of 6.023 x 1023 units of formula (CH3COOH)2. Naturally, this molar mass is larger than the normal formula molar mass. In case of association, Van’t Hoff factor is less than 1, therefore,
MB (abnormal) > MB (normal) when i < 1.
