
Given, radius of atom (r) = 125 pm
(a) For ccp structure, we know that,
Gold (atomic radius = 0.144 nm) crystallises in a face-centred unit cell. What is the length of a side of the cell?
We know that two Na+ ions are replaced by each of the Sr2+ ions while SrCl2 is doped with NaCl. But in this case, only one lattice point is occupied by each of the Sr2+ ions and produce one cation vacancy.
Here 10 – 3 mole of SrCl2 is doped with 100 moles of NaCl Thus, cation vacancies produced by NaCl = 10 – 3 mol Since, 100 moles of NaCl produces cation vacancies after doping = 10 –3 mol
Therefore, 1 mole of NaCl will produce cation vacancies after doping
= 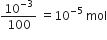
therefore, total cationic vacancies
=10-5 x Avogadro's number
=10-5 x 6.023 x 1023
=6.023 x 10-18 vacancies
