Solution:
We have given that
Gram atomic mass of Cr(M) = 52.0 g mol-1
Edge length of unit cell (a) = 289 pm
Density of unit cell = 7.2 g cm-3
Avogadro's Number
or
Since the unit cell has 2 atoms, it is body centre in nature.
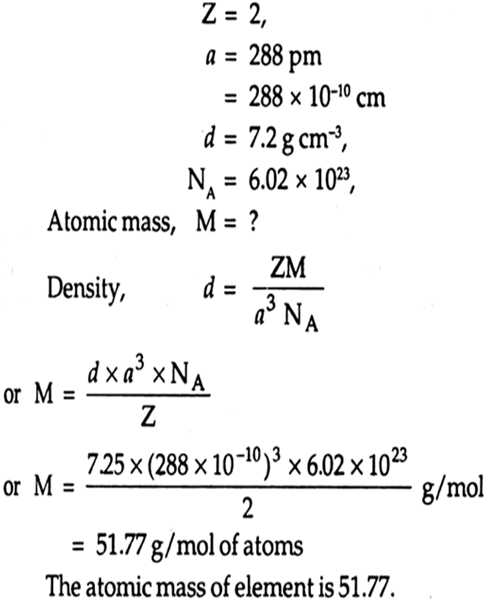
Solution:
We have given that
Unit cell length,
a = 0.40806 nm = 4.086 x 10-10 m
If fcc lattice the number of atoms per unit cell,
i.e. Z = 4
M for Ag = 107.88 g mol-1
For bcc structure
Solution:
We have given that
Density = 10 gm-3
Mass = 100g
edge of unit cell ,a= 4 since it is a Fcc crystal
we have to find total number of atom, So by following relation we can get the result,
Total No. of atoms =
Therefore, number of atoms
thus the number of atoms is 4 x 1031
