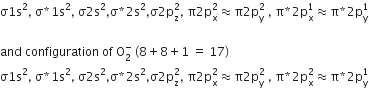Which of the two ions from the list given below have the geometry that is explained by the same hybridization of orbitals
![]()
![]()
![]()
![]()
![]()
D.
![]()
Hybridization of the given molecule is 
Therefore, ![]() both have the same hybridization.
both have the same hybridization.
The Correct order of increasing bond length of C - H, C-O, C - C and C = C is
C - C < C=C < C - O < C - H
C - O < C - H < C - C < C = C
C - H < C - O < C - C < C= C
C - H < C = C < C - O < C - C
D.
C - H < C = C < C - O < C - C
C - H: 0.109 nm
C = C : 0.134 nm
C - O: 0.143 nm
C - C : 0.154 nm
Therefore, Bond length order is
C - H < C = C < C- O < C - C
Which one of the following pairs is isostructural (i.e., having the same shape and hybridization)?
[BCl3 and BrCl3]
[NH3 and NO3-]
[NF3 and BF3]
[NF3 and BF3]
D.
[NF3 and BF3]
If a number of bond pairs and lone pairs are same for the given pairs, they are isostructural.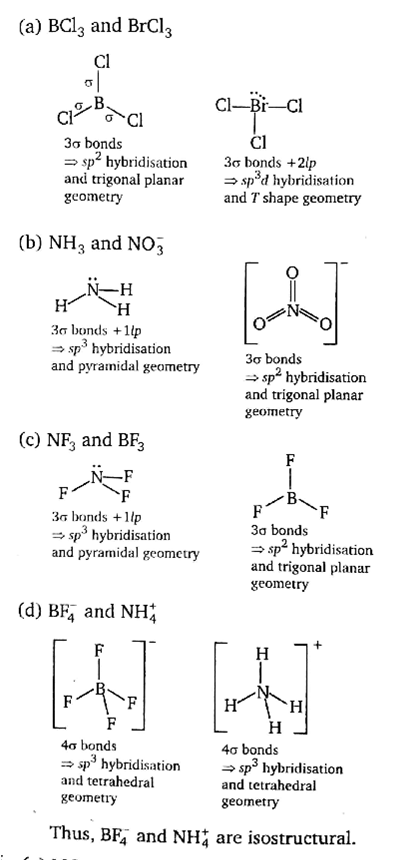
During the change of O2 to O2- ion, the electron adds on which one of the following orbitals?
π* orbital
π orbital
σ* orbital
σ* orbital
A.
π* orbital
During the change of O2 to O2-the electron adds on π* orbital. Molecular orbital configuration of O2 (total e- =16)