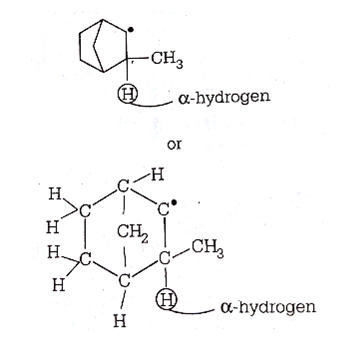The pair of an electron in the given carbanion, is present in which orbital?
is present in which orbital?
sp3
sp2
sp
sp
C.
sp
Consider the molecules CH4, NH3 and H2O. Which of the given statement is false?
The H-O-H bond angle in H2O is larger than the H-C-H bond angle in CH4.
The H-O-H bond angle in H2O is smaller than the H-N-H bond angle in NH3.
The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3
The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3
A.
The H-O-H bond angle in H2O is larger than the H-C-H bond angle in CH4.
According to VBT: lone pair-lone pair repulsion is more than a bond pair -lone pair repulsion.
As the number of lone pair of electrons on central element increases, repulsion between that lone pair of electrons increases and therefore, bond angle decreases.
|
Molecules |
Bond angle |
|
CH4 (no lone pair of electrons) |
1090.5 |
|
NH3(one lone pair of electrons) |
107.50 |
|
H2O (two lone pair of electrons) |
104.450 |
Predict the correct order among the following.
long pair-lone pair> bond pair-bond pair> lone pair>bond pair
bond pair-bond pair> lone pair-bond pair > lone pair -lone pair
lone pair -bond pair > bond pair- bond pair> lone pair -lone pair
lone pair -bond pair > bond pair- bond pair> lone pair -lone pair
D.
lone pair -bond pair > bond pair- bond pair> lone pair -lone pair
According to the VSPER theory, a lone pair occupies more space than a bond pair, Hence when lone pair-lone pair interacts with each other they repel more and thus the correct order is:
lone pair - lone pair > lone pair-bond pair > bond pair -bond pair.
Consider the following compounds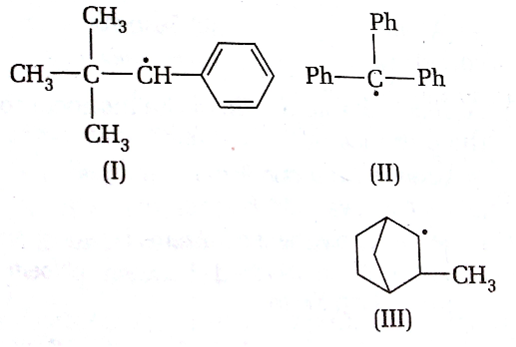
Hyperconjugation occurs in
I only
II only
III only
III only
C.
III only
Hyperconjugation occurs through the H- atoms present on the carbon atom next to the double bond i.e alpha hydrogen atoms. There is no alpha -H in the structure I and II.
So, hyperconjugation occurs in structure III only ie.