The pair of compounds that can exist together is
FeCl3.SnCl2
HgCl2,SnCl2
FeCl2,SnCl2
FeCl2,SnCl2
C.
FeCl2,SnCl2
The compounds with lower oxidation number and which cannot reduce by one another can exist together. Thus, FeCl2 and SnCl2 can exist together as Fe2+ can not be reduced by Sn2+.
Which of the following molecules has the maximum dipole moment?
CO2
CH4
NH3
NH3
C.
NH3
CO2 and CH4 have zero dipole moment as these are symmetrical in nature. Between NH3 and NF3 , NF3 has greater dipole moment though in NH3 and NF3 both, N possesses one lone pair of electrons.
This is because, in the case of NH3, the net N-H bond dipole is in the same direction as the direction of the dipole of the lone pair but in the case of NF3, the direction of the net bond dipole of three -N-F bonds is opposite the that of the dipole of the lone pair.
The enolic form of ethyl acetoacetate as below has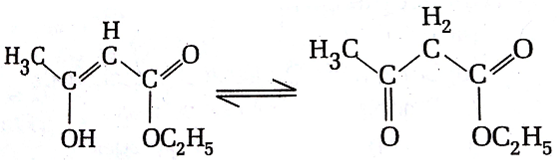
18 sigma bonds and 2 pi-bond
16 sigma bonds and 1 pi-bond
9 sigma bonds and 2 pi-bond
9 sigma bonds and 2 pi-bond
A.
18 sigma bonds and 2 pi-bond
In enolic form of ethyl acetoacetate has 16 single bonds i.e 16 sigma bond and 2 double bonds i.e 2 sigma bonds and 2pi bonds.
Hence, the given structure has 18 sigma bonds and 2 pi bonds.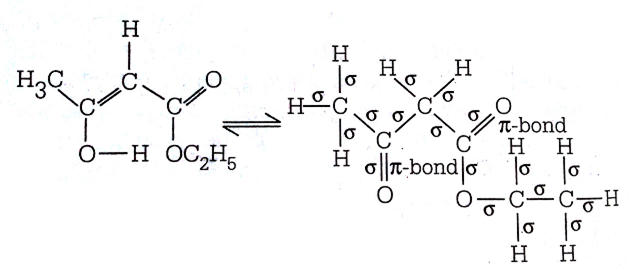
Which one of the following species has the plane triangular shape?
N3
NO3-
NO2-
NO2-
B.
NO3-
Species with sp2 hybridization are plane triangular in shape. Among the given species NO3- si sp2 hybridised with no lone pair of electrons on the central atom, N. whereas, N3, NO2- and CO2 are sp hybridised with a linear shape.