Be2+ is isoelectronic with which of the following ions?
H+
Li+
Na+
Na+
B.
Li+
Isoelectronic species contain the same number of electrons Be2+ contains 2 electrons. Among the given options, only Li+ contains 2 electrons and therefore, it is isoelectronic with Be2+.
H+ --> no electron; Na+ --> 10e-
Li+ --> 2e-;
Mg2+ --> 10 e-
Hence, Be2+ is isoelectronic with Li+
Solubility of the alkaline earth's metal sulphates in water decreases in the sequence
Mg>Ca>Sr>Ba
Ca>Sr>Ba>Mg>
Sr>Ca>Mg>Ba
Sr>Ca>Mg>Ba
A.
Mg>Ca>Sr>Ba
The solubility of the sulphates. The sulphates becomes less soluble as you fo down the group i.e
Mg> Ca>Sr> Ba
The magnitude of the lattice energy remains almost constant as the size of the sulphate ion is so big that small increase in the size of the cation from Be to Ba does not make any difference. However, the hydration energy decreases from Be2+ to Ba2+ appreciably as the size of the cation increases down the group. The significantly high solubility of MgSO4 is due to high enthalpy of solvation on the smaller Mg2+ ions.
The number of d- electrons in Fe2+ (Z = 26) is not equal to the number of electrons in which one of the following?
S- electronic in Mg (Z=12)
p-electrons in Cl( Z=17)
d- electrons in Fe (Z=26)
d- electrons in Fe (Z=26)
B.
p-electrons in Cl( Z=17)
Electronic configuration of Fe2+ is [Ar]3d6 4s0
therefore, Number of electrons = 6
Mg- 1s2 2s2 2p6 (6s electrons)
It matches with the 6d electrons of Fe2+
Cl- 1s2 2s2 2p6 3s2 3p5 (11 p electrons)
It does not match with the 6d electrons of Fe2+
Fe- [Ar] 3d6 4s2 (6d electrons)
It does not match with the 6d electrons of Fe2+
Ne- 1s2 2s2 2p6 3s2 3p 6 (6p electrons)
It matches with the 6d electrons of Fe2+.
Hence, Cl has 11 p electrons which do not match in number with 6d electrons of Fe2+
Which of the following pairs of ions are isoelectronic and isostructural?
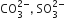
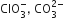


D.

Isostructural chemical compounds have similar chemical structures.
Isoelectronic terms refer to two atom, ions or molecules that have the same number of valence electrons.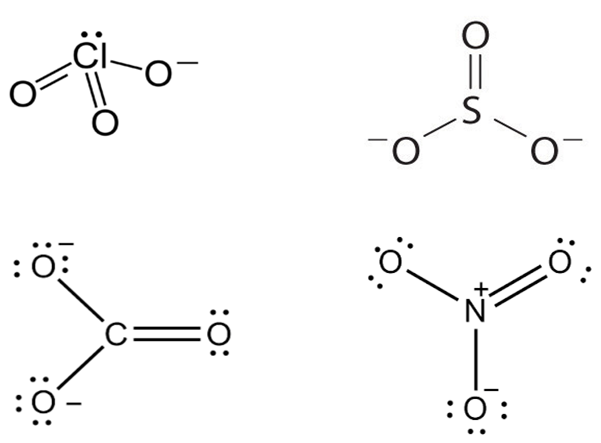
ClO3- = SO32-
Number of electrons
CO32- = 6+2+24 =32
SO32- = 16+2+24 = 32
ClO32- = 17+24+1 = 42
NO32- = 7+2+24 = 33
Hence ClO32- and SO32- are isoelectronic and are pyramidal in shape.
which of the following orders of ionic radii is correctly represented?
H- > H >H+
Na+ >F- >O2-
F- > O2->Na+
F- > O2->Na+
A.
H- > H >H+
It is known that radius of the cation is always smaller than that of the neutral atom due to decrease in the number of orbits.Whereas, the radius of the anion is always greater than a cation due to a decrease in effective nuclear charge. Hence, the correct order is
H- > H >H+
