Dissolving NaCN in de-ionised water will result in a solution having
pH < 7
pH = 7
pOH = 7
pH > 7
D.
pH > 7
Since, on dissolving NaCN in de-ionised water, following reaction takes place.
CN- + H2O HCN + OH-
CN- ions come from the salt of strong base and weak acid.
The solution becomes basic and pH > 7.
PbCl2 is insoluble in cold water. Addition of HCl increases its solubility due to
formation of soluble complex anions like [PbCl3]-
oxidation of Pb(II) to PB(IV)
formation of [Pb(H2O)6]2+
formation of polymeric lead complexes
A.
formation of soluble complex anions like [PbCl3]-
PbCl2 reacts with HCl as follows-
PbCl2 (s) + 2Cl- [PbCl3]- (aq)
PbCl2 (s) + 2Cl- [PbCl4]-2 (aq)
Thus, addition of excess amount of Cl- ions changes the PbCl2 as a soluble complex of [PbCl4]-2. Hence, it becomes soluble.
An alkali is titrated against an acid with methyl orange as indicator, which of the following is a correct combination?
| Base | Acid | End Point |
| Strong | Strong | Pink to colourless |
| Base | Acid | End Point |
| Weak | Strong | Colourless to pink |
| Base | Acid | End Point |
| Strong | Strong | Pinkish Red to Yellow |
| Base | Acid | End Point |
| Weak | Strong | Yellow to Pinkish Red |
D.
| Base | Acid | End Point |
| Weak | Strong | Yellow to Pinkish Red |
Methyl orange is the weak organic base. It is used in the titration of WB vs SA
In a basic medium, equilibrium lies in the backward direction and therefore it shows yellow colour.
In acidic medium, equilibrium shifts in forwarding direction and therefore, colour changes from yellow to red.
The predominant form of histamine present in human blood is (pKa, Histidine = 6.0)
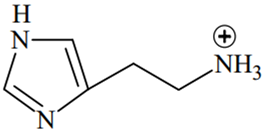
A.

The N-atoms present in the ring will have the same pKa values (6.0), while N atom outside the ring will have different pKa value (pKa > 7.4)
Therefore, two N-atoms inside the ring will remain in the unprotonated form in human blood because of their pKa(6.0) < pH of blood (7.4), while the N-atom outside the ring will remain in protonated form because of its pKa > pH of blood (7.4).
