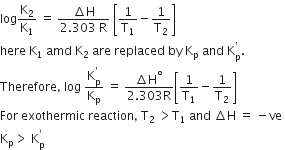The Ksp of Ag2CrO4, AgCl, AgBr and AgI are respectively, 1.1 x 10-12, 1.8 x 10-11, 8.3 x 10-17. Which one of the following salts will precipitate last if AgNO3 solution is added to the solution containing equal moles of NaCl, NaBr, NaI and Na2CrO4?
AgI
AgCl
AgBr
AgBr
D.
AgBr
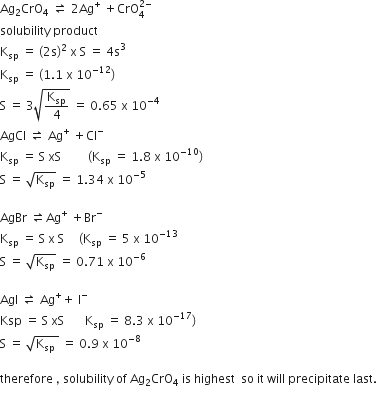
For the reversible reaction,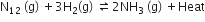
the equilibrium shifts in the forward direction
by increasing the concentration of NH3 (g)
by decreasing the pressure
by decreasing the concentrations of N2 (g) and H2(g)
by decreasing the concentrations of N2 (g) and H2(g)
D.
by decreasing the concentrations of N2 (g) and H2(g)
Any change in the concentration, pressure and temperature of the reaction results in a change in the direction of equilibrium.This change in the direction of equilibrium in governed by Le-Chatelier's principle. According to Le-Chatelier's principle, the equilibrium shifts in the opposite direction to undo the change.
Increasing pressure and decreasing temperature
On increasing pressure, equilibrium shifts in the forward direction where the number of moles decreases while on decreasing temperature. it will move in a forward direction where temperature increases.
Which of the following salts will give highest pH in water?
KCl
NaCl
Na2CO3
Na2CO3
C.
Na2CO3
The highest pH refers to the basic solution containing OH- ions. Therefore, the basic salt releasing OH- ions on hydrolysis will give highest pH in water.
Only the salt of a strong base and weak acid would release OH-ion on hydrolysis. Among the given salts, Na2CO3 corresponds to the basic salt as it is formed by the neutralisation of NaOH [strong base] and H2CO3 [weak acid].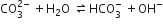
Which of these is least likely to act as a lewis base?
CO
F-
BF3
BF3
C.
BF3
Electron rich species are called lewis base. Among the given, BF3 is an electron deficient species, so have a capacity of electrons accepting instead of donating that's why it is least likely to act as a lewis base. It is a lewis acid.
For a given exothermic reaction Kp and Kp' are the equilibrium constant at temperatures T1 and T2 respectively. Assuming that heat of reaction si constant in temperature range between T1 and T2 it is readily observed that
Kp> Kp'
Kp< Kp'
Kp = Kp'
Kp = Kp'
A.
Kp> Kp'
The equilibrium constant at two different temperatures for a thermodynamic process is given by