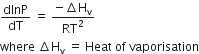MY and NY3, two nearly insoluble salts. have the same Ksp values of 6.2 x 10-13 at room temperature. Which statement would be true in regard to MY and NY3 ?
The molar solubility of MY in water is less than that of NY3
The salts MY and NY3 are more soluble in 0.5 M KY than in pure water
The addition of the Salt of KY to the solution of MY and NY3 will have no effect on their solubilities.
The addition of the Salt of KY to the solution of MY and NY3 will have no effect on their solubilities.
A.
The molar solubility of MY in water is less than that of NY3
For MY,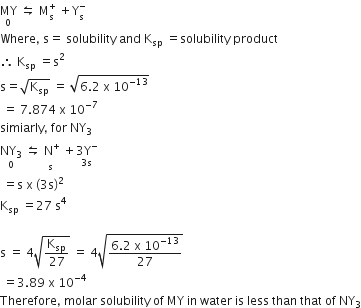
Which one of the following characteristics is associated with adsorption?
ΔG, ΔH and ΔS all are negative
ΔG and ΔH are negative but ΔS is positive
ΔG and ΔS are negative but ΔH is positive
ΔG and ΔS are negative but ΔH is positive
A.
ΔG, ΔH and ΔS all are negative
Adsorption is a spontaneous process that occurs with the release of energy and decrease in the entropy of the substance. For a spontaneous process, ΔG must be negative,
ΔG = ΔH - T ΔS
As the process is exothermic and randomness of the molecule (entropy) decreases hence both ΔH and ΔS will be negative as well.
The addition of a catalyst during a chemical reaction alters which of the following quantities ?
Internal energy
Enthalpy
Activation energy
Activation energy
C.
Activation energy
A catalyst is a substance which alters the reaction but itself remains unchanged in the chemical reaction. In a chemical reaction, it provides a new reaction path by the lowering the activation energy barrier.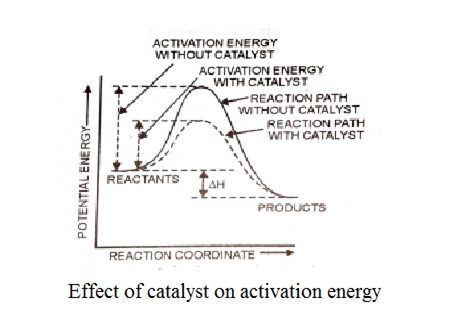
If the value of an equilibrium constant ofr particular reaction is 1.6 x1012 then at equilibrium the system will contains
all reactants
mostly reactants
mostly products
mostly products
C.
mostly products
For a reaction,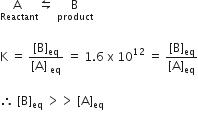
So, mostly the product will be present in the equilibrium mixture.
Consider the following liquid-vapour equilibrium
Which of the following relations is correct?
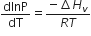

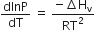
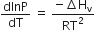
C.
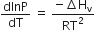
The given phase equilibria is 
This equilibrium states that, when liquid is heated, it converts into vapour but on cooling, it further converts into liquid, which is derived by Clausius clapeyron and the relationship is written as,