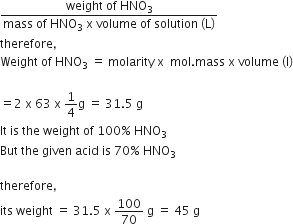Which one of the following statements is not true?
Concentration of DO below 6 ppm is good for the growth of fish
Clean water would have a BOD value of less than 5 ppm
Oxides of sulphur, nitrogen and carbon are the most widespread air pollutant
Oxides of sulphur, nitrogen and carbon are the most widespread air pollutant
A.
Concentration of DO below 6 ppm is good for the growth of fish
The growth of fish is inhibited if the concentration of DO is below 6 ppm.
Mole fraction of the solute in a 1.00 molal aqueous solution is
0.0177
0.0344
1.7700
1.7700
A.
0.0177
1.00 molal aqueous solution = 1.0 mole in 1000 g water nsolute = 1; Wsolvent = 1000g
nsolvent = 1000/18 = 55.56
Xsolute = 1/ 1+ 55.56 = 0.0177
6.02 x 1020 molecules of urea are present in 100 mL of its solution. The concentration of solution is
0.02 M
0.01 M
0.001 M
0.001 M
B.
0.01 M
Given, number of molecules of urea =6.02 x 1020
therefore ,Number of moles
= 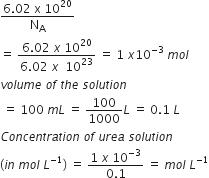
How many grams of the concentrated nitric solution should be used to prepare 250 mL o 2.0M HNO3 ? The concentrated acid is 70% HNO3.
45.0 g conc. HNO3
90.0 g conc. HNO3
70.0 g conc. HNO3
70.0 g conc. HNO3
A.
45.0 g conc. HNO3
Given, molarity of solution = 2
Volume of solution = 250 mL = 250/1000 = 1/4 L
Molar mass of
HNO3 = 1+14+3 x 16 = 63 g mol-1
therefore, Molarity
=