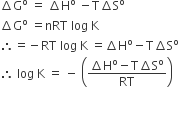The standard reduction potentials for Zn2+/ Zn, Ni2+/ Ni, and F2+/ Fe are –0.76, –0.23 and –0.44 V respectively. The reaction X + Y2+ → X 2+ + Y will be spontaneous when
X = Ni, Y = Fe
X = Ni, Y = Zn
X =Fe, Y= Zn
X =Fe, Y= Zn
D.
X =Fe, Y= Zn
X = Zn, Y = Ni
Zn + Ni2+ →Zn2+ + Ni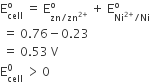
The entropy change involved in the isothermal reversible expansion of 2 moles of an ideal gas from a volume of 10 dm3 to a volume of 100 dm3 at 27°C is
38.3 J mol-1 K-1
35.8 J mol-1 K-1
32.3 J mol-1 K-1
32.3 J mol-1 K-1
A.
38.3 J mol-1 K-1
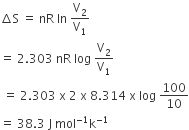
The standard enthalpy of formation of NH3 is– 46.0 kJmol–1. If the enthalpy of formation of H2 from its atoms is – 436 kJ mol–1 and that of N2 is – 712 kJ mol–1,the average bond enthalpy of N – H bond is NH3 is
-964 kJ mol-1
+352 kJ mol-1
+1056 kJ mol-1
+1056 kJ mol-1
B.
+352 kJ mol-1
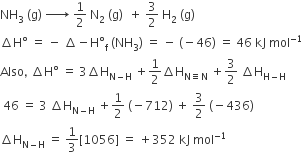
The incorrect expression among the following is

In isothermal process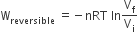


C.

Option C has incorrect expression. The correct expression is,