(I)H2O2 +O3 --> H2O +2O2
(II) H2O2 +Ag2O +--> 2Ag +H2O +O2
Role of hydrogen peroxide in the above reaction is respectively
oxidising in (I) and reducing (II)
reducing (I) and oxidizing in (II)
reducing in (I) and (II)
reducing in (I) and (II)
A.
oxidising in (I) and reducing (II)
In the reaction,
since H2O2 oxidise, O3 into O2 thus it behaves as an oxidising agent.
the further reaction, in the reaction,
Here H2O2 reduces Ag2O into metallic silver [Ag] (as oxidation number is reducing from +1 to 0).Thus, H2O2 behaves as a reducing agent.
When Cl2 gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from
zero to +1 and zero to -5
zero to -1 and zero to +5
zero to -1 and zero to +5
zero to -1 and zero to +5
B.
zero to -1 and zero to +5
When chlorine gas reacts with hot and concentrated NaOH solution, it disproportionates into Chloride (Cl-) and Chlorate (ClO3-) ions. 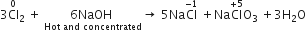
Balance the following equation by chossing the correct options:
xKNO3 + yC12H22O11 → pN2 + qCO2 + rH2O + sK2CO3
| x | y | p | q | r | s |
| 36 | 55 | 24 | 24 | 5 | 48 |
| x | y | p | q | r | s |
| 48 | 5 | 24 | 36 | 55 | 24 |
| x | y | p | q | r | s |
| 24 | 24 | 55 | 48 | 5 |
| x | y | p | q | r | s |
| 24 | 48 | 36 | 24 | 5 | 55 |
B.
| x | y | p | q | r | s |
| 48 | 5 | 24 | 36 | 55 | 24 |
48KNO3 + 5C12H22O11 → 24N2 + 36CO2 + 55H2O + 24K2CO3
A mixture of potassium chlorate, oxalic acid and sulphuric acid is heated. During the reaction which element undergoes a maximum change in the oxidation number?
S
H
Cl
Cl
C.
Cl
When a mixture of potassium chlorate, oxalic acid and sulphuric acid is heated, the following reaction occurs: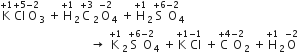
Thus, Cl is the element which undergoes a maximum change in the oxidation state.
