What type of mixtures are separated by the technique of cyrstallisation?
Crystallization is used to purify salt that we get from sea water and to obtain pure substance from their impure samples.
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Mass of sodium chloride = 36g
Mass of solution =100g
Mass of the solution = 100+36g =136g
Concentration of the solution = 36/136 x 100 =26.47%
Differentiate between homogenous and heterogeneous mixtures with examples.
|
s.no |
Homogenous mixture |
Heterogeneous mixture |
|
1. |
It has a uniform composition throughout its mass |
It has no uniform composition.
|
|
2. |
It has no visible boundary or boundaries of separation between its constituents. For example solution of sugar and salt. |
It has visible boundary or boundaries of separation. For example mixture of sugar and sand. |
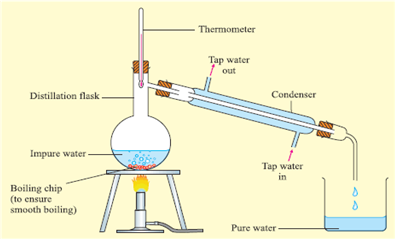
How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than  ), which are miscible with each other?
), which are miscible with each other?
We can separate a mixture containing kerosene and petrol by simple distillation because kerosene and petrol do not decompose on heating and their boiling points are sufficiently apart. The apparatus used for the simple distillation is given below.