 Short Answer Type
Short Answer TypeAssign reasons for the following:
(i) Copper (I) ion is not known in aqueous solution.
(ii) Actinoids exhibit greater range of oxidation states than Lanthanoids.
Stability in the aqueous medium depends on the hydration energy of ions when they attract to water to water molecules. In aqueous solution, Cu+ ion undergoes oxidises and reduces simultaneously
in aqueous to give Cu and Cu2+ ion.

The relative stability of different oxidation states can be seen from their
Electrode potentials 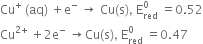
Due to more reduction electrode potential value of Cu+, it undergoes oxidation reaction quite feasible. Hence, the copper (I) ion is not known in aqueous solution.
(ii) The actinoids show a larger number of oxidation states because of the very small energy gap between the 5f, 6d and 7s subshells. The energies are decided on the basis of the (n+1) rule.
The (n+1) values of the three orbitals are under:
5f=5+3=8
6d=5+2=8
7s=7+ 1=8
All of the value comes to be same. Hence they have the same energy.
Silver crystallizes in face-center cubic unit cell. Each side of this unit cell has a length of 400 pm. Calculate the radius of the silver atom. (Assume the atoms just touch each other on the diagonal across the face of the unit cell. That is each face atom is touching the four corner atoms.)
Nitrogen pentoxide decomposes according to equation: 2N2O5(g)---> 4NO2(g) + O2(g)
This first order reaction was allowed to proceed at 40° C and the data below were collected:
|
[N2O5] (M) |
Time (min) |
|
0.400 |
0.00 |
|
0.289 |
20.0 |
|
0.209 |
40.0 |
|
0.151 |
60.0 |
|
0.109 |
80.0 |
(a) Calculate the rate constant. Include units with your answer.
(b) What will be the concentration of N2O5 after 100 minutes?
(c) Calculate the initial rate of reaction.
Explain how the phenomenon of adsorption finds application in each of the following processes:
(i) Production of vacuum
(ii) Heterogeneous catalysis
(iii)Froth Floatation process
Describe the principle behind each of the following processes:
(i)Vapour phase refining of a metal.
(ii)Electrolytic refining of a metal.
(iii)Recovery of silver after silver ore was leached with NaCN.
Write the name, stereochemistry and magnetic behavior of the following:
(At. nos. Mn = 25, Co = 27, Ni = 28)
(i) K4[Mn (CN)6]
(ii) [Co (NH3)5Cl]Cl2
(iii) K2[Ni (CN)4]
