 Short Answer Type
Short Answer TypeDraw the structures of the following :
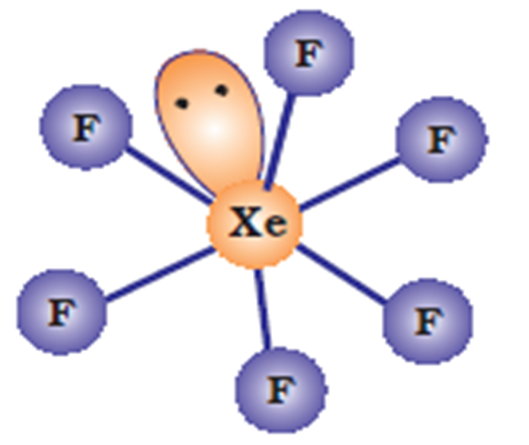
XeF6
Calculate the degree of dissociation (a) of acetic acid if its molar conductivity (Λm) is 39.05 S cm2mol–1. Given λo(H+) = 349.6 S cm2 mol–1 and λo(CH3COO–) = 40.9 S cm2 mol–1
A 10% solution (by mass) of sucrose in water has the freezing point of 269.15 K.Calculate the freezing point of 10% glucose in water if freezing point of pure water is273.15 K.
Given : (Molar mass of sucrose = 342 g mol–1) (Molar mass of glucose = 180 g mol–1)
Calculate the mass of Ag deposited at cathode when a current of 2 amperes was passed through a solution of AgNO3 for 15 minutes.
(Given : Molar mass of Ag = 108 g mol–1 1F = 96500 C mol–1)
Why a solution of [Ni(H2O)6]2+ is green while a solution of [Ni(CN)4]2– is colourless? (At. no. of Ni = 28)
