 Multiple Choice Questions
Multiple Choice QuestionsThe most probable radius (in pm) for finding the electron in He+ is
0.0
52.9
26.5
105.8
C.
26.5
Most probable radius = a0/Z
where a0 = 52.9 pm. For helium ion, Z = 2
rmp = = 26.45 pm
For the reaction of one mole of zinc dust with one mole of sulphuric acid in a bomb calorimeter, U and w corresponds to
U < 0, w = 0
U < 0, w < 0
U > 0, w = 0
U > 0, w > 0
For reaction, 2NOCl (g) 2NO (g) + Cl2 (g), Kc at 427°C is 3 × 10-6 L mol-1. The value of Kp is nearly
7.50 × 10-5
2.50 × 10-5
2.50 × 10-4
1.75 × 10-4
For the chemical equilibrium,
CaCO3(s) CaO(s) + CO2(g)
can be determined from which one of the following plots?
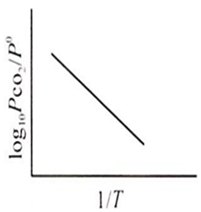
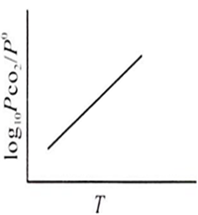
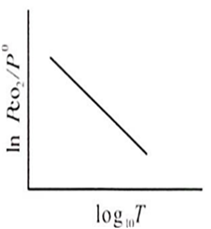
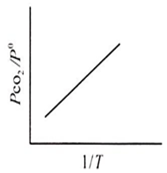
Assertion : Potassium ferrocyanide is diamagnetic whereas potassium ferricyanide is paramagnetic.
Reason : Crystal field splitting in ferrocyanide ion is greater than that of ferricyanide ion.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : SeCl4 does not have a tetrahedral structure.
Reason : Se in SeCl4 has two lone pairs.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : First ionization energy for nitrogen is lower than oxygen.
Reason : Across a period effective nuclear charge decreases.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : B2 molecule is diamagnetic.
Reason : The highest occupied molecular orbital is of σ type.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
In the balanced chemical reaction
IO3- + aI- + bH+ → cH2O + dI2
a, b, c and d respectively corresponds to
5, 6, 3, 3
5, 3, 6, 3
3, 5, 3, 6
5, 6, 5, 5
Among the following pairs of ions, the lower oxidation state in aqueous solution is more stable than the other, in
Tl+, Tl3+
Cu+, Cu2+
Cr2+, Cr3+
V2+, VO2+
