 Multiple Choice Questions
Multiple Choice Questions1.00 g of a non- electrolyte solute (molar mass 250 g mol-1) was dissolved in 51.2 g of benzene. If the freezing point depression constant, Kf of benzene is 5.12 K Kg mol-1, the freezing point of benzene will be lowered by:
0.4 K
0.3 K
0.5 K
0.5 K
A.
0.4 K
Molality of non- electrolyte solute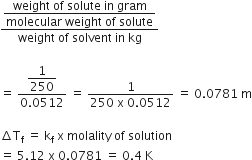
A solution of acetone in ethanol:
shows a negative deviation from Raoult's law
shows a positive deviation from Raoult's law
behave likea near ideal solution
behave likea near ideal solution
A hypothetical electrochemical cell is shown below
A- | A+ (xM)|| B+ (yM)|B+
The emf measured is +0.20 V. The cell reaction is:
A+ + B → A + B+
A+ + e- → A ; B+ + e- → B-
the cell reaction cannot be predicted
the cell reaction cannot be predicted
During osmosis, flow of water through a semi-permeable membrane is:
from a solution having higher concentration only
from both sides of a semi-permeable membrane with equal flow rates
from both sides of a semi-permeable membrane with unequal flow rates
from both sides of a semi-permeable membrane with unequal flow rates
The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2 D). This is because:
In NH3 as well as in NF3 the atomic dipole and bond dipole are in the same direction
In NH3 the atomic dipole and bond dipole are in opposite directions
In NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directions
In NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directions
The number of unpaired electrons in a paramagnetic diatomic molecule of an element with atomic number 6 is:
2
3
4
4
Which one of the following orders is not in accordance with the property stated against it?
F2 > Cl2 > Br2 > I2 : Oxidising power
HI> HBr> HCl >HF: Acidic property in water
F2 > Cl2 > Br2 > I2: Electrongeativity
F2 > Cl2 > Br2 > I2: Electrongeativity
In a set of reactions, propionic acid yielded a compound D.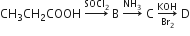
The structure of D would be:
CH3CH2CH2NH2
CH3CH2CONH2
CH3CH2NHCH3
CH3CH2NHCH3
