 Multiple Choice Questions
Multiple Choice QuestionsThe reaction
![]()
is called
Claisen-Schmidt reaction
Kolbe-Schmidt reaction
Schmidt reaction
Kolbe's reaction
A.
Claisen-Schmidt reaction
C.
Schmidt reaction
Schmidt Reaction refers to an organic chemical reaction wherein azides are reacted with the carbonyl group of a compound to give rise to amines or amides. This reaction was first reported by Karl Friedrich Schmidt in 1924.
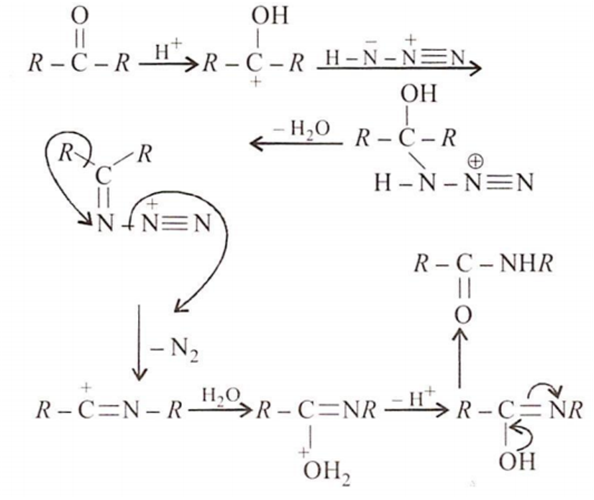
Assertion : (CH3)3CCOC(CH3)3 and acetone can be distinguished by the reaction with NaHSO3.
Reason : HSO3- is the nucleophile in bisulphite addition.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Cyclohexanone exhibits keto-enol tautomerism.
Reason : In cyclohexanone, one form contains the keto group (>C=O) while other contains enolic group (- C=C- OH).
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion: Phenol is more reactive than benzene towards electrophilic substitution reaction.
Reason : In the case of phenol, the intermediate carbocation is more resonance stabilised.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Benzaldehyde is more reactive than ethanal towards nucleophilic attack.
Reason : The overall effect of -I and +R effect of phenyl group decreases the electron density on the carbon of C=O atom group in benzaldehyde.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Teflon has high thennal stability and chemical inertness.
Reason : Teflon is a thermoplastic.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion: Anilinium chloride is more acidic than ammonium chloride.
Reason : Anilinium chloride is resonance stabilised.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : 2-Butanol on heating with H2SO4 gives 1-butene and 2-butene.
Reason : Dehydration of 2-butanol follows Saytzeff's rule.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Ethers behave as bases in the presence of mineral acids.
Reason : It is due to the presence of lone pair of electrons on the oxygen.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
