 Multiple Choice Questions
Multiple Choice QuestionsConsidering the state of hybridization of carbon atoms, find out the molecule among the following which is linear.
CH3 - C ≡ C - CH3
CH2 = CH - CH2 - C ≡ CH
CH3 - CH2 - CH2 - CH3
CH3 - CH2 - CH2 - CH3
A.
CH3 - C ≡ C - CH3
CH3 - C ≡ C - CH3 linear, because C2 and C3 are sp hybridised.
The Lassaigne's extract is boiled with conc HNO3 while testing for halogens. By doing so it
helps in the precipitation of AgCl
increases the solubility product of AgCl
increases the concentration of NO3- ions
increases the concentration of NO3- ions
Which one of the following is present as an active ingredient in bleaching powder for bleaching action?
Ca(OCl)2
CaO2Cl
CaCl2
CaCl2
Name the type of the structure of silicates in which one oxygen atom of [SiO4]4- is shared?
Sheet silicate
Pyrosilicate
Three-dimensional silicate
Three-dimensional silicate
The correct IUPAC name of the compound of 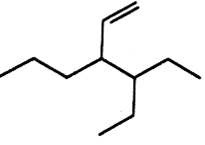
3 - ethyl - 4 ethenyl heptane
3 - ethyl - 4 - propylhex- 5-ene
3-(1-ethyl propyl) hex - 1 ene
3-(1-ethyl propyl) hex - 1 ene
Standard electrode potential of three metal X, Y and Z are -1.2 V, +0.5 V and -3.0 V respectively. The reducing power of these metals will be
Y > X > Z
Z> X> Y
X > Y > Z
X > Y > Z
The freezing point depression constant for water is -1 . 86o C m-1. If 5.00 g Na2SO4 is dissolved in 45.0 g H2O, the freezing points is changed by -3.82oC. Calculate the van't Hoff factor for Na2SO4.
2.63
3.11
0.381
0.381
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of +2 oxidation state will be there in which of the following order?
(At. no. Cr = 24, Mn = 25, Fe = 26, Co = 27)
Fe > Mn > Co> Cr
Co > Mn > Fe > Cr
Cr > Mn > Co > Fe
Cr > Mn > Co > Fe
