 Multiple Choice Questions
Multiple Choice QuestionsThe type of hybridisation in SF6, molecule is
sp3d
dsp3
sp3d
d2sp3
C.
sp3d
S(16 e-) ground state = 1s2, 2s2, 2p6, 3s2, 3p4
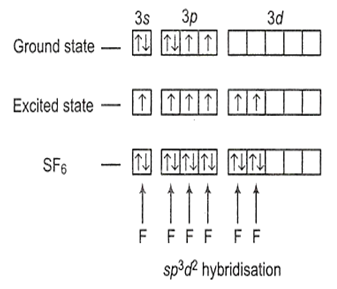
Therefore, the type of hybridisation in SF6 molecule is sp3d2.
Among LiCl, BeCl2, BCl3 and CCl4 the covalent bond character follows the order
LiCl < BeCl2 < BCl3 < CCl4
BCl3 < CCl4 < BeCl2 < LiCl
BeCl2 < LiCl < CCl4 < BCl3
CCl4 < BCl3 < BeCl2 < LiCl
Maximum number of electrons in a subshell of an atom determined by the following?
4l + 2
2n2
4l - 2
2l + 1
pKa of acetic acid and pKb of ammonium hydroxide are 4.76 and 4.75 respectively. Calculate the pH of ammonium acetate solution.
6.02
7.005
8
5.602
The value of Kc for the reaction,
2A B + C is 2 x 10-3. At a given time, if the composition of reaction mixture is [A] = [B] = [C] = 3 x 10-3 M. Which is true?
The reaction will proceed in forward direction
The reaction will proceed in backward direction
The reaction will proceed in any direction
None of the above
Number of hydrogen-bonded water molecules associated in CuSO4.5H2O is
one
two
three
All the five
Which of the following species do not show disproportionation on reaction?
ClO-, Cl , Cl and Cl
Cl
Cl
ClO-
None of these
During estimation ofnitrogen in the organic compound by Kjeldahl's method, the ammonia evolved from 0.5 g of the compound in Kjeldahl's estimation of nitrogen, neutralised 10 mL of 1M H2SO4. Find out the percentage of nitrogen in the compound.
14%
28%
56%
68%
On addition of conc. H2SO4 to a chloride salt, colourless fumes are evolved but in case of iodide salt, violet fumes come out. This is because
H2SO4 reduces HI to I2
HI is of violet colour
HI gets oxidised to I2
HI changes to HIO3
