 Multiple Choice Questions
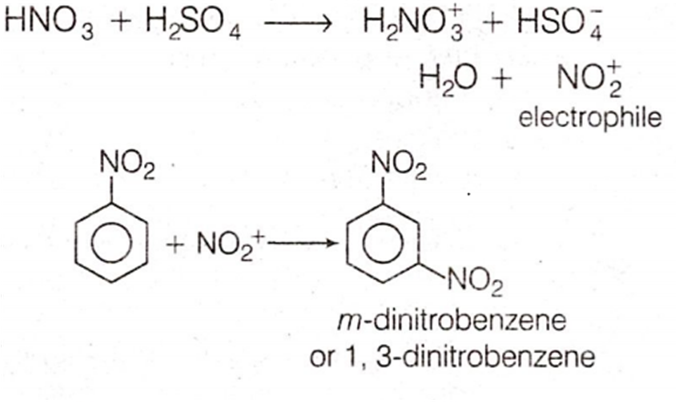
Multiple Choice QuestionsNitrobenzene on reaction with conc. HNO3/H2SO4 at 80-100°C forms which one of the following products?
1, 2-dinitrobenzene
1, 3-dinitrobenzene
1, 4-dinitrobenzene
1, 2, 4-trinitrobenzene
B.
1, 3-dinitrobenzene
NO2 group being electron withdrawing reduces electron density at output positions. Hence, now the meta-position becomes electron rich on which the electrophile (nitronium ion) attacks during nitration.
Some meta-directing substituents in aromatic substitution are given. Which one is most deactivating?
The radical  because it has
because it has
6p-orbitals and 6 unpaired electrons
7p-orbitals and 6 unpaired electrons
7p-orbitals and 7 unpaired electrons
6p-orbitals and 7 unpaired electrons
Which of the following compounds will not undergo Friedal-Craft's reaction easily?
Cumene
Xylene
Nitrobenzene
Toluene
What is the activation energy for a reaction if its rate doubles when the temperature is raised from 20°C to 35C ? (R = 8.314 J mol-1 K-1).
342 kJ mol-1
269 kJ mol-1
34.7 kJ mol-1
15.1 kJ mol-1
A hydrogen gas electrode is made by dipping platinum wire in a solution of HCl of pH=10 and by passing hydrogen gas around the platinum wire at 1 atm pressure. The oxidation potential of electrode would be
0.059 V
0.59 V
0.118V
1.18V
A reaction having equal energies of activation for forward and reverse reactions has
S = 0
G = 0
H = 0
H = G = S = 0
At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1cm2 mol-1 and at infinite dilution its molar conductance is 238 ohm-1cm2 mol-1. The degree of ionisation of ammonium hydroxide at the same concentration and temperature is
2.080%
20.800%
4.008%
40.800%
