Advertisement
Explain the formation of covalent bonds in (i) chlorine molecule; (ii) carbon tetrachloride and (iii) ammonia.
(i) Formation of chlorine molecule: Two chlorine atoms share one electron of each and acquire stable electronic configuration as shown:
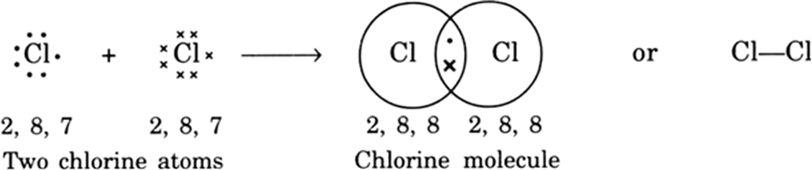
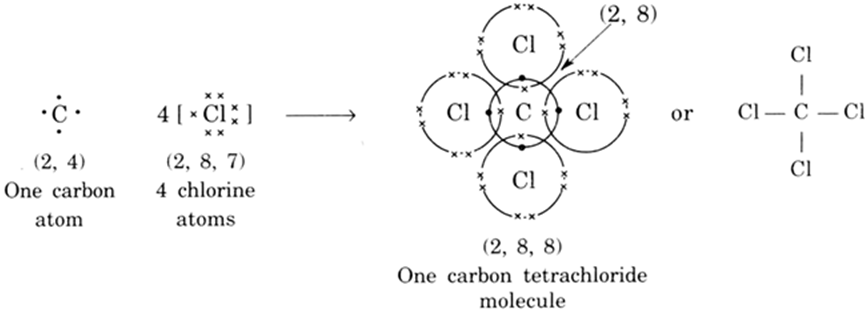
(ii) Formation of carbon tetrachloride: Carbon atoms has four electrons in the outermost shell. In the formation of carbon tetrachloride, it shares four electrons with four chlorine atoms. In turn four chlorine atoms share one electron each with one carbon atom. Thus in all, one carbon atom and four chlorine atoms complete their octet by mutual sharing of electrons and carbon tetrachloride containing four covalent bonds is formed as shown:
(iii) Formation of ammonia molecule: Nitrogen (N) atom has 5 electrons (2, 5) in the outermost shell. It shares one electron each of three hydrogen atoms to form ammonia molecule as shown:
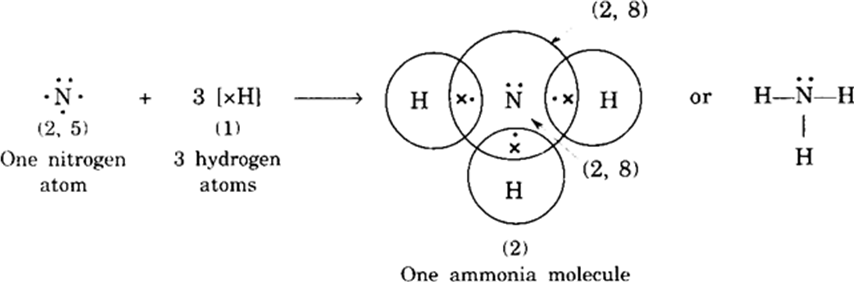
One unshared pair of electrons in ammonia molecule is not involved in bond formation and is called a lone pair of electrons.
4450 Views
Advertisement
Soaps produce __________ while synthetic detergents produce ___________ with hard water.
Discuss the reactivity of carbon in relation to its electronic configuration.
Soaps are the alkali salts of ______________.
Sodium lauryl sulphate is an example of ______________.
Advertisement