Advertisement
What is meant by double bond or double covalent bond? Give some examples.
The bond between two atoms formed by sharing of two pairs of electrons is called a double bond. Two horizontal lines between two atoms denote a double bond, e.g., O = O(O2)
(i) Formation of oxygen molecule, O2: Oxygen atom has six electrons in its outermost shell, two short of forming a complete octet. Thus each oxygen atom in O2 shares 2 electrons with the other oxygen atom and attains stable configuration as shown: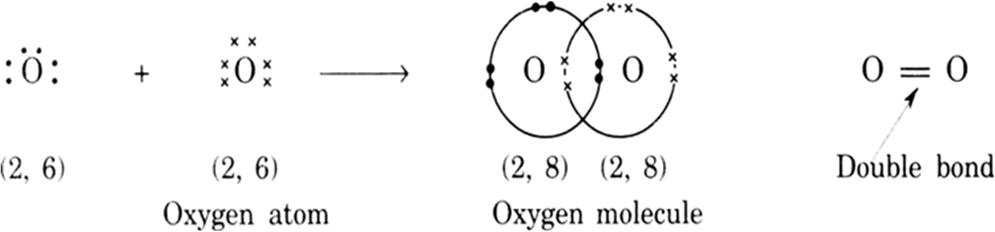
(ii) Formation of ethylene molecule (C2H4): In ethylene two carbon atoms share two pairs of electrons between them and the rest two electrons on each carbon atom share one electron each with two hydrogen atoms. Thus ethylene molecule has one double bond (C = C) and four single bonds (C—H).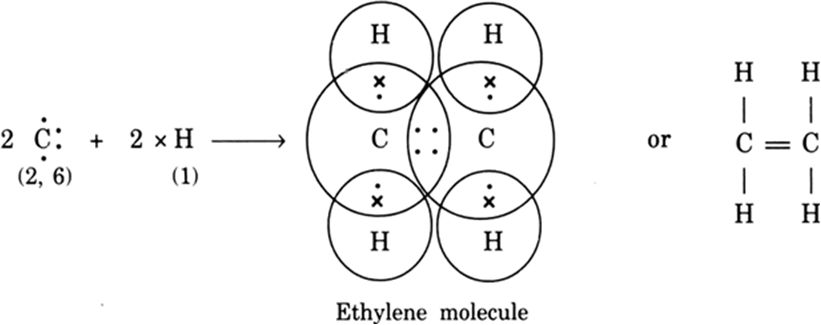
1331 Views
Advertisement
Glacial acetic acid is:
- an aqueous solution of alcohol
- vinegar
- an aqueous solution of acetic acid
- 100% pure ethanoic acid.
Taking a simple example, explain how the formation of covalent bond between two atoms brings stability to the molecule formation.
What is a covalent bond?
What is triple bond or triple covalent bond? Explain the formation of triple bond giving two examples.
Advertisement