Advertisement
Taking a simple example, explain how the formation of covalent bond between two atoms brings stability to the molecule formation.
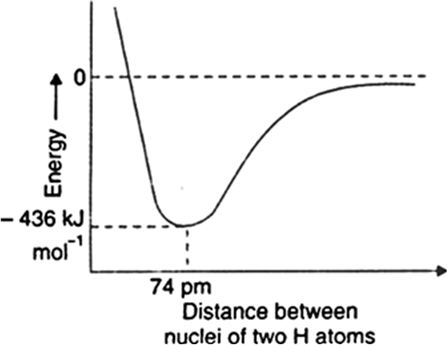
Take the example of formation of hydrogen molecule. When two hydrogen atoms come close together, nucleus of one atom exerts an attractive force on the electrons of the other atom and vice versa to complete their octet. This brings the two atoms still closer and potential energy decreases. At a particular distance between the two atoms, potential energy becomes minimum. Hydrogen atoms cannot come further closer because then the repulsive forces come into operation and cause the energy to increase. This stage of minimum energy which binds the two hydrogen atoms to form a molecule is actually the covalent bond.
1050 Views
Advertisement
What is meant by double bond or double covalent bond? Give some examples.
What is triple bond or triple covalent bond? Explain the formation of triple bond giving two examples.
Glacial acetic acid is:
- an aqueous solution of alcohol
- vinegar
- an aqueous solution of acetic acid
- 100% pure ethanoic acid.
What is a covalent bond?
Advertisement