Advertisement
A solution of copper sulphate was kept in an iron pot. After a few days, the iron pot was found to have a number of holes in it. Write the equation of the reaction that took place. Explain this reaction in terms of reactivity.
Iron is more reactive than copper and so iron displaces copper from copper sulphate solution. Since iron is being consumed, holes appear in the pot. On the other hand, Cu2+ ions in solution change into copper metal.
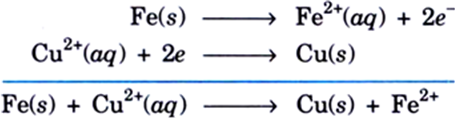
1482 Views
Advertisement
Why should we not throw small sodium pieces into a sink in the laboratory?
How would you show that silver is chemically less reactive than copper?
In nature aluminium is found in the form of its compounds while gold is found in free state. Give reasons.
Explain the terms:
(a) Anodising
(b) Aqua regia.
Advertisement