Advertisement
Explain with the help of a diagram the effect of change in concentration of solution on the molar conductance of (i) a weak electrolyte, (ii) a strong electrolyte.
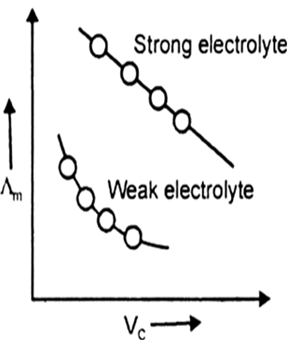
Λm increase a little in strong electrolyte because numbers of ions do not increase appreciably, only mobility of ions increases. Λm increases sharply in weak electrolyte because both number of ions and mobility of ions increases.
126 Views
Advertisement
With the halp of ionic equations describe what happen: When
(i) pH of a solution of dichromate ions is raised.
(ii) Potassium manganate is electro-chemically oxidised.
(i) pH of a solution of dichromate ions is raised.
(ii) Potassium manganate is electro-chemically oxidised.
The standard reduction potential values of three metal cations Xa+, Yb+ and Zc+are + 0.52, – 3.03, – 1.18 V respectively. Arrange the corresponding metals, in the order of their increasing reducing power.
State two advantages of H2O fuel cell over ordinary cell.
Explain why:
(i) E° for Mn3+ / Mn2+ couple is more positive than that Fe3+/Fe2+. (At. No. Mn = 25, Fe = 26).
(ii) Ce3+ can be easily oxidised to Ce4+ (At. No. Ce= 58).
(i) E° for Mn3+ / Mn2+ couple is more positive than that Fe3+/Fe2+. (At. No. Mn = 25, Fe = 26).
(ii) Ce3+ can be easily oxidised to Ce4+ (At. No. Ce= 58).
Advertisement