Advertisement
Calculate the emf of the cell Zn/Zn2+ (0.1 M) || Cd2+ (0.01 M) | Cd at 298 k. (given)
E°Zn2+/Zn = – 0.76 V and E°Cd2+/Cd = – 0.40 V).
Zn/Zn2+(0.1 M) || Cd2+ (0.01) | Cd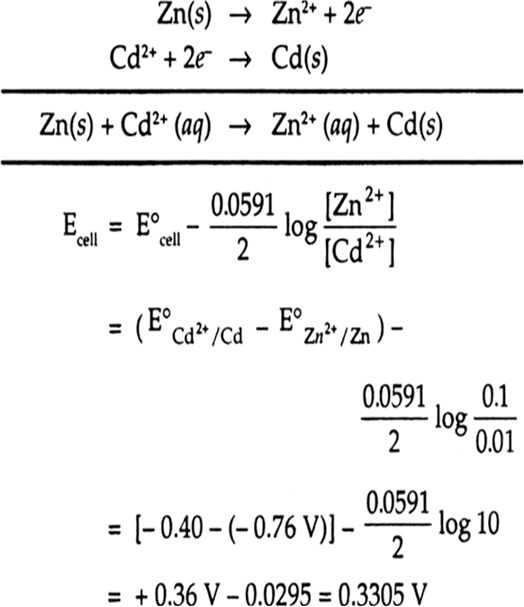
1204 Views
Advertisement
The following curve is obtained when molar conductivity λm (y-axis) is plotted against the square root of concentration C1/2 (x-axis) for two electrolytes A and B.
(a) What can you about the nature of the two electrolytes A and B.
(b) How do you account for the increase in molar conductivity λm for the electrolytes A and B on dilution.
How is the standard free energy change related to
(i) emf of a galvanic cell related to the reaction.
(ii) equilibrium constant of the reaction in equilibrium state?
(i) emf of a galvanic cell related to the reaction.
(ii) equilibrium constant of the reaction in equilibrium state?
Write the cell reactions which occur in lead storage battery (i) when the battery is in use and (ii) when the battery is on charging.
Prove that the free energy (ΔG) and the emf of an electro-chemical cell are related by
ΔG = – nFE0.
ΔG = – nFE0.
Advertisement