Advertisement
Why should a solution of a non-volatile solute boil at a higher temperature ? Draw the diagram to prove your point?
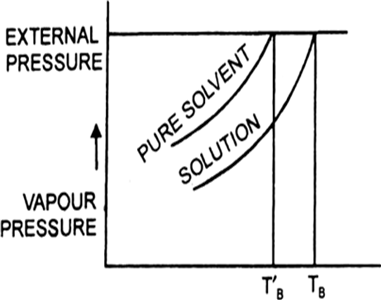
Boiling point is that temperature at which the vapour pressure of the solution becomes equal to the external pressure since on adding a nonvolatile solute, the vapour pressure of the solution gets lowered, therefore, it will boil at a higher temperature.
160 Views
Advertisement
Illustrate elevation in boiling point with the help of vapour pressure temperature curve of a solution. Show that elevation in boiling point is a colligative property?
(a) Draw a labelled diagram to show the change in vapour pressure of a solvent, when a non-volatile solute is added to it.
(b) Show that the change in boiling point of the solvent in this diagram?
Why should the solution of a nonvolatile solute freeze at a lower temperature? Draw a neat diagram to prove your point.
Which type of deviation is shown by the solution formed by mixing cyclohexane and ethanol?
Advertisement