Advertisement
(a) Draw a labelled diagram to show the change in vapour pressure of a solvent, when a non-volatile solute is added to it.
(b) Show that the change in boiling point of the solvent in this diagram?
(a) It is found that the boiling point of the solution is always higher than that of the pure solvent. The elevation in boiling point is the increase in boiling point when a non-volatile solute is added to a solvent.
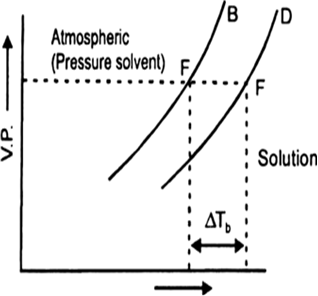
(b) In the above diagram ΔTb indicates the change (increase) in boiling point of solvent.
177 Views
Advertisement
Why should the solution of a nonvolatile solute freeze at a lower temperature? Draw a neat diagram to prove your point.
Why should a solution of a non-volatile solute boil at a higher temperature ? Draw the diagram to prove your point?
Illustrate elevation in boiling point with the help of vapour pressure temperature curve of a solution. Show that elevation in boiling point is a colligative property?
Which type of deviation is shown by the solution formed by mixing cyclohexane and ethanol?
Advertisement