Advertisement
Explain the nature of the covalent bond using the bond formation in CH3Cl.
Covalent bond/s is formed by sharing of electrons so that the combining atom/s complete their outermost shell. In CH3Cl, this happens as follows:
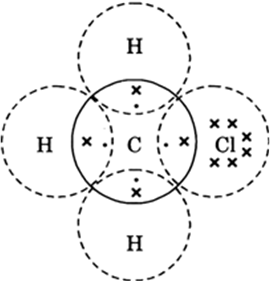
Three hydrogen atoms complete (K = 1 + 1 = 2) their shells by sharing three electrons (one electron each) of carbon atom. Chlorine complete its outer shell (L = 7 + 1 = 8) by sharing its one out of seven electrons with one electron of carbon atom. Thus carbon atom shares in all its four electrons with three of three hydrogen atoms and one of chlorine atom and completes its outer shell (L = 4 + 3 + 1 = 8).
272 Views
Advertisement
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that:
- the food is not cooked completely
- the fuel is not burning completely
- the fuel is wet
- the fuel is burning completely.
Draw the electron dot structures for:
(a) ethanoic acid
(b) H2S
(c) propanone
(d)F2’
What is a homologous series? Explain with an example.
How can ethanol and ethanoic acid be differentiated
on the basis of their physical and chemical properties.
on the basis of their physical and chemical properties.
Advertisement