Advertisement
How will you distinguish between the following pairs of terms:
Tetrahedral void and octahedral void?
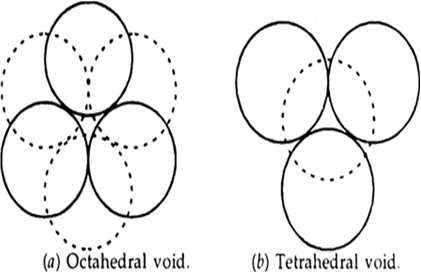
The void created by six spheres in contact is called an octahedreal void Fig. 1.21. (a) and void created by the four spheres in contact is called a tetrahedral void Fig. 1.21 (b).
337 Views
Advertisement
How can you determine the atomic mass of an unknown metal if you know its density and the dimension of its unit cell? Explain.
How will you distinguish between the following pairs of terms:
Crystal lattice and unit cell?
'Stability of a crystal is reflected in the magnitude of its melting points. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the inter molecular forces between these molecules?
How will you distinguish between the following pairs of terms:
Hexagonal close-packing and cubic close-packing?
Advertisement