 Multiple Choice Questions
Multiple Choice QuestionsLithium has a bcc structure. Its density is 530 kg m-3 and its atomic mass is 6.94 g mol-1. Calculate the edge length of a unit cell of lithium metal. (NA = 6.02 x 1023 mol-1).
352pm
527pm
264pm
264pm
A given metal crystallises out with a cubic structure having edge length of 361 pm. If there are four metal atoms in one unit cell, what is the radius of one atom.
40 pm
127 pm
80 pm
80 pm
If a is the length of the side of a cube, the distance between the body centred atom and one corner atom in the cube will be
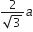
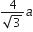
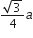
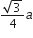
A metal has a fcc lattice. The edge length of the unit cell is 404 pm. The density of the metal is 2.72 g cm-3. The molar mass of the metal is (NA Avogadro's constant= 6.02 x 1023 mol-1)
40 g mol-1
30 g mol-1
27 g mol-1
27 g mol-1
Which of the following statements about the interstitial compounds is incorrect?
They retain metallic conductivity
They are chemically reactive
They are much harder than the pure metal
They are much harder than the pure metal
A metal crystallises with a face-centered cubic lattice. The edge of the unit cell is 408 pm. The diameter of the metal atom is
288 pm
408 pm
144 pm
204 pm
The number of octahedral voids (s) per atom present in a cubic close-packed structure is
1
3
2
4
A structure of a mixed oxide is cubic close packed (ccp). The cubic unit cell of mixed oxide is composed of oxide ions. One fourth of the tetrahedral voids are occupied by divalent metal A and the octahedral voids are occupied by a monovalent metal B. The formula of the oxide is
ABO2
A2BO2
A2B3O4
A2B3O4
The correct statement regarding defects in the crystalline solid is
Schottky defects have no effect on the density of crystalline solid
Frenkel defect decreases the density of crystalline solids
Frenkel defect is a dislocation defect
Frenkel defect is a dislocation defect
