Arrange the following compounds in order of decreasing acidity
-
II>IV>I>III
-
I>II>III>IV
-
III>I>II>IV
-
III>I>II>IV
C.
III>I>II>IV
Electron withdrawing group increases the acidic strength of the compounds by destabilising and stabilising the phenoxide ion formed respectively.
The presence of an electron withdrawing group increases the acidic strength by stabilising the phenoxide ion. On other hands, the presence of electron releasing group destabilises the phenoxide ion and decrease the acidic strength.
NO2>Cl>CH3>OCH3
An aromatic compound 'A' of molecular formula C7H7ON undergoes a series of reactions as shown below. Write the structures of A, B, C, D and E in the following reactions: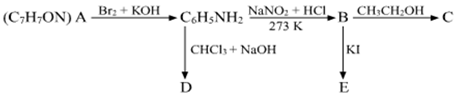
A solution of (–) –1 – chloro –1 – phenylethane is toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of
-
carbanion
-
Carbene
-
Carbocation
-
Carbocation
Select the ether among following that yields methanol as one the products on reaction with cold hydroiodic acid
1-methoxybutane
1-methoxybutane-2-methylpropane
2-methoxy-2-methylpropane
methoxybenzene
Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Ethanal and Propanal
(ii) Benzoic acid and Phenol