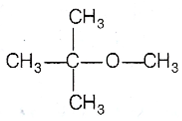
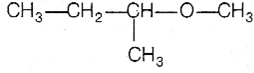Multiple Choice Questions
Multiple Choice QuestionsMethanol and ethanol are miscible in water due to
covalent character
hydrogen bonding character
oxygen bonding character
None of the above
When 23 g sodium metal reacts with methyl alcohol , it will form :
1 moles of H2
2 mole of H2
0.5 mole of H2
All of the above
Which one of the following forms propanenitrile as the major product?
Propyl bromide + alcoholic KCN
Ethyl bromide + alcoholic KCN
Ethyl bromide + alcoholic AgCN
Propyl bromide + alcoholic AgCN
The reaction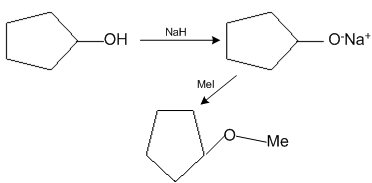
Can be classified as
Alcohol formation reaction
Dehydration reaction
Williamson alcohol synthesis reaction
Williamson alcohol synthesis reaction
The reaction,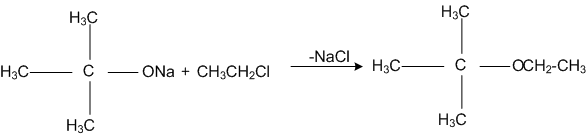
is called,
Williamson synthesis
Williamson continuous etherification process
Etard reaction
Gattermam -Koch reaction
Which of the following will not be soluble in sodium hydrogen carbonate ?
2,4,6 trinitrophenol
Benzoic acid
o-nitrophenol
o-nitrophenol
Among the following sets of reactants which one produces anisole?
CH3CHO;RMgX
C6H5OH;NaOH; CH3I
C6H5OH;neutral FeCl3
C6H5OH;neutral FeCl3
Among the following ethers, which one will produce methyl alcohol on treatment with hot concentrated HI?